Ever wonder why your antidepressant takes weeks to work, or why you can’t eat aged cheese while on certain antibiotics? It’s not magic. It’s chemistry. Every pill, injection, or patch you take is a carefully designed chemical that interacts with your body in precise, measurable ways. Understanding how medicines work isn’t just for doctors-it’s the key to using them safely, avoiding dangerous side effects, and knowing when something’s going wrong.
What Actually Happens When You Take a Pill?
When you swallow a tablet, it doesn’t just disappear. It travels through your stomach and intestines, gets absorbed into your bloodstream, and then gets carried to its target. But here’s the catch: most drugs don’t work everywhere. They’re like keys that only fit specific locks in your body. These locks are called receptors-proteins on the surface of cells that control things like pain signals, mood, or blood pressure.
Take aspirin. It doesn’t just numb pain. It blocks an enzyme called COX-1, which makes chemicals that cause inflammation and pain. By shutting down that enzyme, aspirin reduces swelling and fever. SSRIs like fluoxetine (Prozac) work differently. They don’t add serotonin to your brain. Instead, they block the pump that recycles serotonin back into nerve cells. That leaves more serotonin floating around, helping to lift your mood over time.
Some drugs are designed to hit one target. Others? They’re messier. Lithium, used for bipolar disorder, affects at least five different systems in the brain. That’s why it’s so tricky to dose. Too little, and it doesn’t help. Too much, and you get tremors, confusion, or even kidney damage. That’s why blood tests are required-to keep lithium levels between 0.6 and 1.2 mmol/L. Anything outside that range can be dangerous.
Why Some Drugs Are Safe-And Others Aren’t
Safety doesn’t come from a pill being "natural" or "prescribed." It comes from knowing exactly what the drug does and how your body handles it.
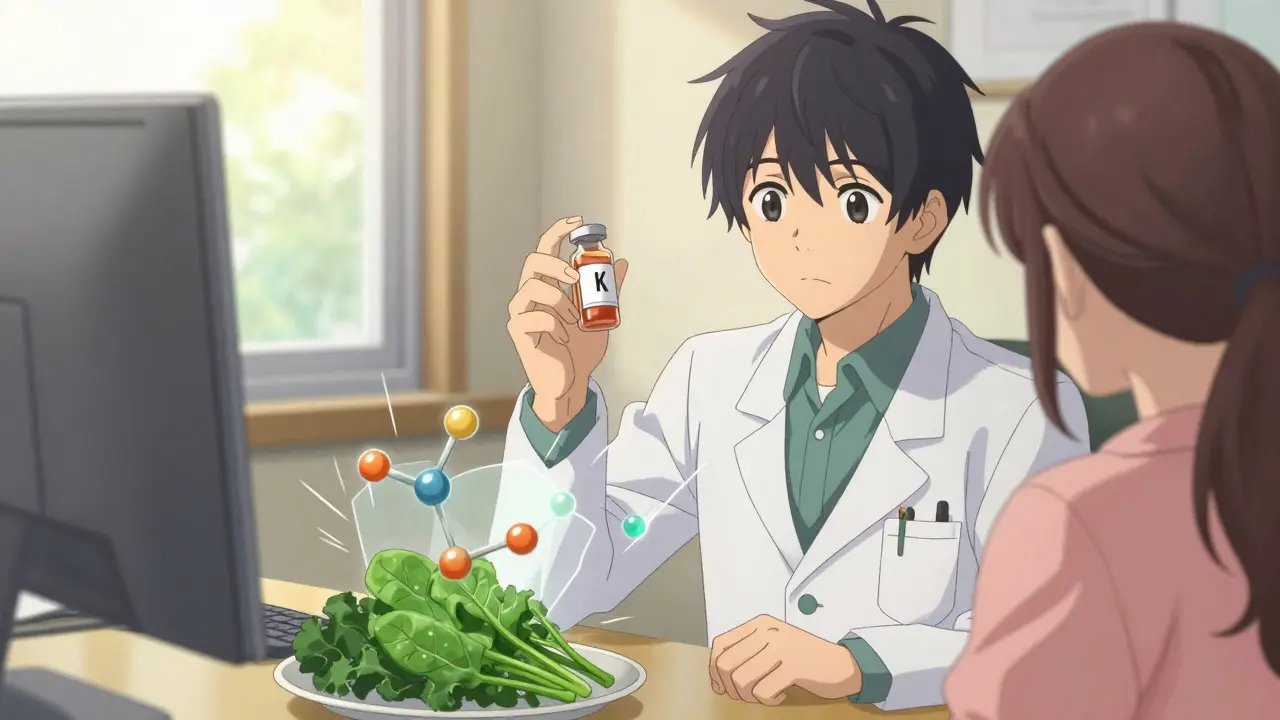
Warfarin, a blood thinner, is a perfect example. It works by blocking vitamin K, which your body needs to make clotting factors. But vitamin K is also in spinach, kale, broccoli-you name it. If you suddenly eat a big salad, your blood starts clotting again. That’s why people on warfarin need steady vitamin K intake. One study found that patients who understood this connection were 60% less likely to have dangerous bleeding episodes.
Then there’s the flip side: drugs with unclear mechanisms. Dimebon, an antihistamine used in Russia for decades, was tested for Alzheimer’s in the 2000s. It showed early promise-until researchers realized they didn’t know how it worked. Without knowing the mechanism, they couldn’t predict side effects or optimize dosing. The trial failed. Meanwhile, trastuzumab (Herceptin) works by targeting a specific protein (HER2) found only on certain breast cancer cells. Before prescribing it, doctors test tumors for HER2. If it’s there? The drug works. If not? It does nothing. That precision makes it safer.
What Your Body Does to the Drug (And Why It Matters)
It’s not just what the drug does to your body. Your body does things to the drug too. This is called pharmacokinetics.
Some drugs get broken down by your liver before they even reach your bloodstream. That’s the first-pass effect. Morphine loses about 30% of its strength this way. Propranolol? Up to 90%. That’s why some pills need higher doses than others.
Then there’s protein binding. About 95-98% of many drugs stick to proteins in your blood. That’s fine-until another drug comes along and kicks them off. Sulfonamide antibiotics can displace warfarin from these proteins. Suddenly, more warfarin is free and active. Your blood thins too much. Bleeding risk spikes. This isn’t rare. The FDA reports that over 30% of adverse drug events involve these kinds of interactions.
The blood-brain barrier is another gatekeeper. Most drugs can’t cross it. But for Parkinson’s, levodopa is specially designed to sneak through. It’s converted into dopamine inside the brain. If it couldn’t cross that barrier, it would be useless. That’s why some medications are formulated as combinations-like Sinemet®-to get past biological walls.
How Understanding Your Medicine Keeps You Safe
Patients who understand how their medication works are less likely to have bad outcomes.
A 2023 survey from the American Cancer Society found that 78% of patients on trastuzumab who understood its HER2-targeting mechanism could spot early signs of heart damage-like shortness of breath or swelling-before it became serious. Only 29% of those who didn’t understand the mechanism could do the same.
On Reddit’s r/Pharmacy community, a thread with over 4,000 upvotes showed how patients on warfarin used their knowledge of vitamin K to adjust meals-not to avoid greens, but to keep intake consistent. One man said, "I eat one cup of spinach every Tuesday. No more, no less. I know why now. I sleep better."
Even statins, often blamed for muscle pain, are safer when patients understand their mechanism. Statins block HMG-CoA reductase, an enzyme your liver uses to make cholesterol. But that same enzyme is also involved in muscle function. When patients know this, they report muscle aches earlier-before they turn into rhabdomyolysis, a rare but life-threatening condition. Data from Drugs.com shows those patients were 3.2 times more likely to catch the warning signs.
When Medications Become Dangerous
Some dangers aren’t obvious. MAO inhibitors, used for depression, stop your body from breaking down tyramine-a chemical in aged cheese, wine, and cured meats. If you eat these while on an MAOI, tyramine builds up. Your blood pressure spikes. You could have a stroke. The FDA’s MedWatch database recorded dozens of emergency room visits from this interaction in 2022. All preventable. All tied to lack of education.
Thalidomide is the worst-case scenario. In the 1950s, it was sold as a safe morning sickness pill. But one version of the molecule (an enantiomer) caused severe birth defects. The other version helped with nausea. Back then, scientists didn’t know how to separate them. Today, we do. That’s why new drugs are tested for stereochemistry-every mirror-image version of a molecule. It’s now standard.
Even modern drugs aren’t foolproof. Natalizumab (Tysabri), used for multiple sclerosis, blocks immune cells from entering the brain. Sounds good. But it also lets a rare virus, JC virus, run wild in the brain, causing progressive multifocal leukoencephalopathy (PML). The FDA now requires doctors to complete training on this risk before prescribing it. Why? Because they understood the mechanism-and knew the danger.
What’s Changing in Medication Safety
Things are getting smarter. The FDA’s "Pharmacology 2030" initiative is pushing for every new drug to have its mechanism fully mapped. By 2025, they’ll roll out 15 new biomarker tests to monitor safety in real time. For example, if you’re on an EGFR inhibitor for lung cancer, a skin rash isn’t just a side effect-it’s a sign the drug is working. Doctors now use rash severity to adjust doses.
The NIH’s "All of Us" program is collecting genetic data from a million people to see how genes affect drug response. Early results show 28% of adverse reactions are tied to genetic differences in how people metabolize drugs. Someone might need half the dose of a standard medication because their liver processes it too fast-or too slow.
By 2028, some hospitals may use "digital twins"-computer models of your body based on your genes, weight, liver function, and more. These models will simulate how a drug will behave in you before you even take it. Early tests at Mayo Clinic show they can cut adverse events by up to 60%.
But here’s the hard truth: 30% of medications currently prescribed still have unknown mechanisms. That’s why 1.3 million people end up in U.S. emergency rooms every year because of drug side effects. And it costs $3.5 billion annually. Knowledge isn’t power here. It’s a lifeline.
What You Can Do Right Now
- Ask your pharmacist: "How does this drug work?" Don’t accept "It just helps." Push for a simple explanation.
- Write down one thing your medication does to your body-and one thing your body does to the medication.
- Check for food or supplement interactions. Green vegetables, alcohol, grapefruit, and St. John’s wort are common culprits.
- If you’re on a drug with a narrow safety window (like lithium, warfarin, or digoxin), know your target range. Ask for a copy of your lab results.
- Track symptoms. A new muscle ache, rash, or mood change could be your body telling you something’s off.
Medicines aren’t magic bullets. They’re tools. And like any tool, they’re safest when you understand how they work.
Why do some medications take weeks to work?
Many drugs, especially those for mental health like SSRIs, don’t work by instantly flooding your system. Instead, they trigger slow changes in brain chemistry. For example, fluoxetine blocks serotonin reuptake, but your brain needs time to adapt by making new receptors and adjusting signaling pathways. This process takes 3-6 weeks. That’s why you shouldn’t stop if you don’t feel better right away-your body is still adjusting.
Can I take two medications if I don’t know how they interact?
No. Even over-the-counter drugs can be dangerous when combined. For example, ibuprofen and warfarin together increase bleeding risk. Antacids can block absorption of antibiotics like ciprofloxacin. Always check with a pharmacist before mixing medications-even if they’re from different doctors. There are over 1,000 known drug interactions, and many aren’t listed on labels.
Why do some people need higher doses of the same drug?
It’s often genetic. Some people have liver enzymes that break down drugs faster (fast metabolizers), meaning they need higher doses. Others process drugs slowly (slow metabolizers) and can get sick on standard doses. The FDA now recommends genetic testing for certain drugs like clopidogrel and codeine. If you’ve had side effects before, ask about pharmacogenetic testing-it could prevent future problems.
Is a "natural" supplement safer than prescription medicine?
Not necessarily. St. John’s wort, often used for mild depression, can interfere with birth control, antidepressants, and even chemotherapy. Garlic supplements can thin your blood like aspirin. Herbal products aren’t tested for safety the way FDA-approved drugs are. Just because something is natural doesn’t mean it’s safe-or that it won’t interact with your prescription.
What should I do if I miss a dose?
It depends on the drug. For antibiotics, missing a dose can let bacteria survive and become resistant. For blood thinners like warfarin, missing a dose can make your blood too thick. For SSRIs, skipping a day can cause withdrawal symptoms like dizziness or nausea. Always check the medication guide or call your pharmacist. Never double up unless instructed. A general rule: if it’s been less than half the time until your next dose, take it. Otherwise, skip it and resume your schedule.







Jacob den Hollander
Man, I wish I'd known all this when I was on Zoloft for two years and thought I was just "broken." It's not magic, it's chemistry-and that's actually kind of beautiful. My brain didn't "fail," it just needed time to rewire. I started keeping a little journal: "Today I took my pill. Today my body is adjusting." It helped me not panic when nothing changed for weeks.
Also, I eat one cup of kale every Sunday. Not because I'm healthy, but because I read somewhere that warfarin folks need consistency. I don't even like kale. But I do it. And I sleep better. Weird, right?
Jessica Klaar
This is the kind of post that makes me believe people can actually learn stuff if it's explained right. I used to think "natural" meant safe-until my aunt went to the ER after mixing St. John’s wort with her blood pressure med. She didn't even know they interacted. I printed out this whole thing and left it on her fridge. No words. Just the article. She texted me three days later: "Okay, I get it now."
Also, I'm starting to track my meds. One thing my body does to the drug? I'm a slow metabolizer. My pharmacist said I'm lucky I didn't have a meltdown on a normal dose of propranolol. So now I take half. And I feel like a genius.
PAUL MCQUEEN
Look, I read the whole thing. It's well-researched. But let’s be real-most people don’t care. They just want a pill that makes them feel better. The fact that 30% of drugs still have unknown mechanisms? That’s not a feature. That’s a bug in the system. And no, "pharmacogenetic testing" isn’t gonna fix a broken healthcare model that prioritizes profit over patient education.
Also, "digital twins"? Cute. I’ll believe it when I see it. Until then, I’m still taking my meds and hoping for the best. Which, honestly, sounds like the American healthcare system in a nutshell.
glenn mendoza
It is with profound appreciation that I acknowledge the depth of insight presented herein. The mechanistic elucidation of pharmacological action-particularly the distinction between pharmacodynamics and pharmacokinetics-is not merely academic, but profoundly human.
That patients who comprehend the biological rationale behind their regimens exhibit improved adherence and reduced adverse events is not surprising; it is a logical consequence of agency. When individuals are granted understanding, they are empowered. When empowered, they become partners in their own care.
May this paradigm become standard. Not as a trend. Not as a marketing slogan. But as a moral imperative.
Randy Harkins
Yessssss 🙌 I’ve been screaming this from the rooftops for years! It’s not about taking pills-it’s about knowing WHY they work. I’m on lamotrigine and I know it stabilizes glutamate and sodium channels. So when I get that weird buzzing in my fingers? I don’t panic. I check my dose. I call my doc. I don’t just wait for it to "get better."
Also, I eat one avocado a day. Not because I’m trendy. Because my pharmacist told me it’s low in vitamin K. And I don’t want to end up like that guy on Reddit who had a stroke because he ate a whole bag of spinach.
Knowledge = power. And power = not dying.
Tori Thenazi
Okay, but what if this is all a lie? What if Big Pharma doesn’t actually want us to understand how drugs work? Because if we did, we’d realize they’re just patching symptoms while the root causes-pollution, stress, trauma, capitalism-go ignored.
And what about the fact that lithium was discovered by accident? And that trastuzumab was almost scrapped because the company thought it was "too niche"? Who even decided what "mechanism" matters? Was it scientists? Or investors?
I’m not saying don’t take your meds. I’m saying: question everything. Even this post. Especially this post.
Angie Datuin
Interesting. I didn’t know warfarin and vitamin K were linked like that. I’ll start keeping track. Thanks.
Camille Hall
I’m a nurse, and I’ve seen too many patients stop their meds because they "didn’t feel anything." This post? It’s the kind of thing I wish every patient got handed with their prescription. Not a leaflet. Not a website link. Just this. Clear. Calm. Real.
Also, I tell my patients: "Your body doesn’t see pills as medicine. It sees them as chemicals. Your job? To help it understand what to do with them." It sounds weird, but it works.
Monica Warnick
So… if I’m on an SSRI and I skip a day, am I basically rewiring my brain backward? Like, is it like a video game where you lose progress? And if I eat grapefruit, does it turn my pill into a bomb? I’m just trying to survive here, y’all.
I don’t want to be a science nerd. I just want to not die.
Ashlyn Ellison
My mom took lithium for 20 years. She never got her levels checked. She just took it. One day, she stopped talking. Went to the hospital. Turned out her kidneys were failing. No one told her. No one tested her. Just "take it."
This post? It’s the thing I wish someone had given her. I’m printing it. I’m mailing it to my doctor. And I’m making my sister read it. No one else should go through that.
Frank Baumann
Let me tell you about my cousin. He was on MAOIs. He ate blue cheese. He didn’t even know. He had a hypertensive crisis. Went into a coma. Spent 11 days in ICU. Now he’s fine. But he’ll never eat cheese again. And he’s terrified of his own fridge.
And guess what? His doctor never warned him. Just handed him the script. Said "avoid tyramine." Didn’t say what that meant. Didn’t list foods. Didn’t even spell it out.
So here’s my question: if your doctor can’t explain it in plain English, why are you taking it? I’m not being dramatic. I’m being terrified. And I’m not alone.
Chelsea Deflyss
you forgot to mention that most drugs are made in china and have 30% filler lol
Tricia O'Sullivan
As someone who has lived with bipolar disorder for over two decades, I can attest that understanding the mechanism of lithium was the single most empowering moment of my medical journey. It transformed me from a passive recipient of care into an active participant. The fact that this post is so meticulously detailed gives me hope that the future of pharmacology will be grounded in clarity-not mystery.
Thank you.