Personalized Drug Reaction Risk Calculator
Assess Your Medication Risk
Enter your personal factors to calculate your risk of adverse drug reactions based on the latest pharmacogenomic research.
Ever taken a medication that worked perfectly for your friend but gave you a terrible reaction? You’re not alone. One person gets dizzy from a common painkiller, another sleeps fine on the same dose. Someone develops a rash from an antibiotic, while their sibling takes it without a hitch. This isn’t bad luck or coincidence - it’s biology. Medications don’t act the same way in every body. The reason? Individual variation in drug side effects is built into our genes, our age, our other medications, and even our diet.
Genes Are the Hidden Switches
Your genes control how your body handles drugs. Think of them like tiny switches that turn enzymes on or off. The most important of these are the cytochrome P450 enzymes - especially CYP2D6, CYP2C9, and CYP2C19. These enzymes break down about 80% of all prescription drugs. But not everyone has the same version of these enzymes. About 5% to 10% of people of European descent are “poor metabolizers” of CYP2D6. That means their bodies can’t break down drugs like codeine, beta-blockers, or some antidepressants efficiently. The drug builds up, and side effects pile up - nausea, dizziness, even heart rhythm problems. On the other end, 1% to 2% of Europeans and up to 29% of Ethiopians are “ultra-rapid metabolizers.” Their bodies clear drugs so fast that the medicine doesn’t work at all. A child given standard-dose codeine for pain might get no relief, while an ultra-rapid metabolizer could turn it into morphine too quickly and risk overdose. Take warfarin, a blood thinner. Two people can get the same prescription, but one bleeds internally while the other clots. Why? Two genes - CYP2C9 and VKORC1 - explain 30% to 50% of why warfarin doses vary so wildly. In 2023, the FDA updated labels for warfarin to include genetic dosing guidance. Hospitals that test for these variants before prescribing cut major bleeding events by 31% and got patients to the right dose 27% faster.Age Changes How Drugs Work
As we age, our bodies change - and not always in ways we notice. Older adults have more body fat and less muscle. Fat-soluble drugs like diazepam or amitriptyline get stored in fat tissue and released slowly, making them last longer and build up. That’s why a 65-year-old might feel groggy on a dose that a 30-year-old handles fine. Kidneys and liver - the main organs that clear drugs - also slow down with age. By 70, kidney function drops by about 50% compared to age 30. That means drugs like metformin or digoxin stick around longer. A common dose becomes a dangerous one. That’s why older adults are 300% more likely to be hospitalized for side effects than younger people, especially when taking five or more medications at once.Other Drugs Can Turn Up the Volume
It’s not just your genes or age. What else you’re taking matters. Many side effects happen because one drug interferes with another. Take amiodarone, a heart rhythm drug. It blocks the enzyme CYP2C9. If you’re on warfarin, amiodarone can make your warfarin levels spike by 100% to 300%. That’s a recipe for internal bleeding. This isn’t rare. About 1 in 5 hospitalizations for side effects involve drug-drug interactions. Even over-the-counter stuff can cause trouble. St. John’s wort, a popular herbal supplement for mood, speeds up CYP3A4 - a liver enzyme that breaks down everything from birth control pills to statins. Someone on birth control who starts taking it might get pregnant. Someone on simvastatin could get severe muscle damage. These interactions are often missed because patients don’t tell their doctors about supplements.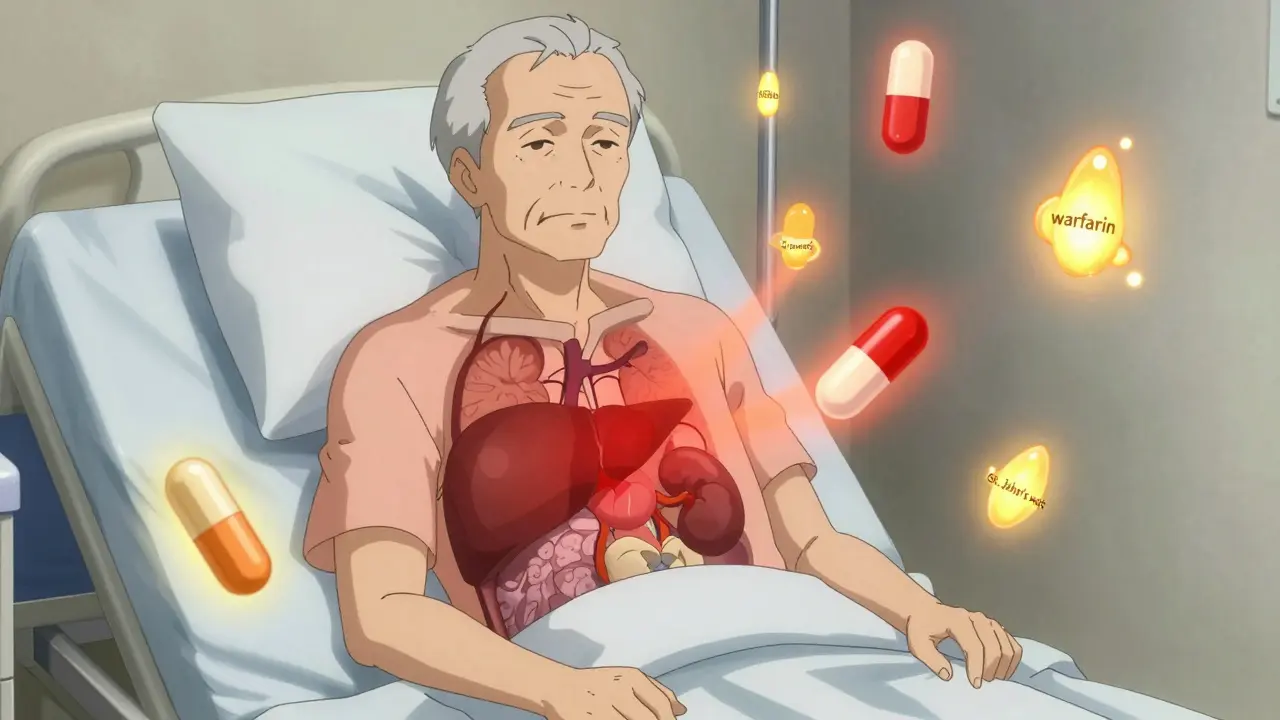
Health Conditions Change the Game
Your current health can alter how drugs behave. Inflammation from an infection, arthritis, or even long-term stress can shut down cytochrome P450 enzymes by 20% to 50%. A person with a cold might handle a painkiller fine normally, but during an infection, that same dose could become toxic. Liver disease? Your body can’t process drugs like acetaminophen properly. Even a normal dose can cause liver failure. Kidney disease? Drugs like ibuprofen or lithium build up fast. A person with untreated kidney disease might need half the dose - or none at all.Genetic Testing Is Here - But It’s Not Everywhere
We’ve known for decades that genes affect drug response. But testing hasn’t become routine - yet. The FDA now includes pharmacogenomic info on over 300 drug labels. For 44 of them, they give specific dosing advice based on genetics. In oncology, testing for TPMT gene variants before giving mercaptopurine to kids with leukemia cut severe toxicity from 25% to just 12%. In psychiatry, testing for CYP2D6 and CYP2C19 helps avoid antidepressants that won’t work or will cause bad side effects. But here’s the catch: only 18% of U.S. insurers cover pharmacogenomic testing. Only 32% of hospitals have systems that automatically flag risky gene-drug combos in electronic records. And 68% of doctors say they don’t feel trained to use the results. A 2023 study found that even when tests are done, most physicians don’t change prescriptions based on them - because they don’t know what to do with the data. The good news? Costs have dropped. In 2015, a full pharmacogenomic panel cost $2,000. Today, it’s around $250. Medicare started covering testing for 17 high-risk drugs in January 2024. Point-of-care tests - like the new CYP2C19 test that gives results in 60 minutes - are now available in ERs and cardiology clinics. This isn’t science fiction anymore. It’s becoming standard care - just slowly.
What This Means for You
If you’ve had a bad reaction to a drug, or if a medication didn’t work for you when it worked for someone else, it might not be your fault. You might be a poor metabolizer. Or you might have a gene variant that makes you extra sensitive. The next time you’re prescribed a new drug, ask: “Could my genes affect how this works?” Talk to your pharmacist. They’re trained to spot drug interactions and can check if your meds might clash. If you’re on multiple drugs, especially if you’re over 65, ask if a pharmacogenomic test could help. It’s not magic - but it can prevent hospital visits. One 2022 study of 10,000 patients found those who got genetic testing had 32% fewer emergency room trips and 26% shorter hospital stays. And if you’re taking something expensive - like a $300-a-month asthma drug - and it’s not helping, ask if you’ve been tested for the 5-LO or LTC4 synthase gene variants. About 15% of severe asthma patients have variants that make these drugs work wonders. The rest? They get no benefit. That’s not just wasted money - it’s wasted time and health.The Future Is Personal
The old model - “one size fits all” - is fading. We’re moving toward precision prescribing. In the next five years, polygenic risk scores - which look at hundreds of genes at once - will replace single-gene tests. These will predict drug response with 40% to 60% more accuracy. By 2030, your electronic health record might auto-suggest the right drug and dose based on your DNA, your age, your kidney function, and your other meds - all in real time. But this future won’t happen unless patients ask for it. If you’ve been told “this drug didn’t work for you” and never got an explanation, you’re part of the problem - and the solution. Demand better. Ask questions. Push for testing. Because your body isn’t broken. It’s just different. And now, we’re finally learning how to listen.Why do some people have side effects from a drug while others don’t?
It comes down to genetics, age, other medications, and health conditions. Some people have gene variants that make them poor or ultra-rapid metabolizers of drugs, meaning their bodies process them too slowly or too quickly. Age affects liver and kidney function, changing how drugs are cleared. Other drugs can block or speed up metabolism, and conditions like liver disease or inflammation can alter how drugs behave. It’s never just one factor - it’s a mix.
Can genetic testing prevent bad drug reactions?
Yes, in specific cases. For example, testing for CYP2C9 and VKORC1 genes before prescribing warfarin reduces major bleeding by 31%. Testing for TPMT before giving mercaptopurine to leukemia patients cuts severe toxicity from 25% to 12%. For clopidogrel, CYP2C19 testing identifies poor metabolizers who won’t benefit from the drug. When used correctly, genetic testing can prevent up to 30% of adverse drug reactions.
Are over-the-counter supplements safe to take with prescription drugs?
Not always. St. John’s wort can make birth control, statins, and antidepressants less effective. Garlic and ginkgo can increase bleeding risk with blood thinners. Even common herbs like ginger or turmeric can interfere with drug metabolism. Many people don’t tell their doctors about supplements - but pharmacists can help spot dangerous interactions. Always disclose everything you’re taking.
Why isn’t genetic testing for drugs more common?
Cost, lack of training, and poor integration into healthcare systems. Only 18% of U.S. insurers cover testing, and 68% of doctors feel unprepared to use the results. Hospitals often don’t have systems that automatically alert clinicians to risky gene-drug combos. Even when tests are done, results aren’t always acted on. But Medicare’s 2024 coverage expansion and point-of-care tests are changing that.
Should I get tested before taking a new medication?
If you’re on multiple medications, over 65, have had bad reactions before, or are taking a high-risk drug like warfarin, clopidogrel, certain antidepressants, or chemotherapy, yes. Ask your doctor or pharmacist if pharmacogenomic testing is right for you. It’s especially valuable if a drug didn’t work in the past or caused side effects. Testing costs as little as $250 now - and could save you from hospitalization.








RAJAT KD
Genetics isn't magic-it's math. CYP2D6 poor metabolizers? That's not 'bad luck,' it's pharmacokinetics. If your doctor prescribes codeine without checking your phenotype, they're not just negligent-they're dangerous.
Angela Stanton
OMG I just realized why that SSRIs made me feel like a zombie 😵💫 but my sister was fine?? CYP2C19 poor metabolizer confirmed. Also, why does no one talk about how St. John’s wort ruined my birth control?? 🤦♀️
Jenci Spradlin
my grandma took warfarin for 12 years and never bled out. they just kept adjusting the dose. genetic testing is cool but if your doc knows what they're doing, you don't need a $250 test. just monitor INR like we did in the 90s.
Kiruthiga Udayakumar
I'm so glad someone finally said this. My mom got hospitalized because her doctor didn't know about CYP2C9 and VKORC1. She was on 5mg, then suddenly 15mg-she almost died. I cried for days. This isn't science fiction-it's survival.
Chris Kauwe
Let me be blunt: this whole pharmacogenomics movement is just Silicon Valley rebranding old science as 'innovation' to sell tests. The FDA's 300 drug labels? Most are advisory, not mandatory. And let’s not forget-only 3% of Americans have even heard of CYP enzymes. We’re not ready for precision medicine. We’re still arguing about masks and vaccines. This is a luxury for the overinsured.
Meanwhile, rural hospitals can’t even get insulin. You want to spend $250 on a gene panel when people are skipping meals to afford metformin? That’s not progress. That’s performative healthcare.
And don’t get me started on the pharma companies pushing these tests. They’re not doing it to save lives-they’re doing it to lock you into their branded drugs. If your CYP2D6 status says you're a poor metabolizer, guess what? They’ve got a $400/month alternative. Coincidence? I think not.
Real solution? Train every damn pharmacist. Pay them to review scripts. Stop outsourcing safety to DNA. We’ve had clinical judgment for centuries. Don’t outsource it to a 23andMe-style spit kit.
And yes-I know you’re gonna say 'but it reduces ER visits!'-so does not giving people 12 different meds at once. Maybe the problem isn’t your genes. Maybe it’s your polypharmacy problem.
Jacob Paterson
Wow. So the solution to bad drug reactions is... asking your doctor nicely? How revolutionary. Meanwhile, I’m still waiting for the day when my insurance covers the test instead of making me beg for it like I’m asking for a loan. Oh wait-I forgot. In America, your genes only matter if you can afford to know them.
Pooja Kumari
I just want to say... I cried reading this. Not because I’m emotional (though I am) but because I’ve been this person. I took fluoxetine for 3 years, felt like a ghost, and my doctor kept saying 'it's just adjusting.' But my cousin took the same pill and said it 'saved her life.' Why? Why me? Why not her? I finally got tested last year-CYP2C19 poor metabolizer. I was so angry. All those months of numbness, the weight gain, the suicidal thoughts... it wasn't me. It was my enzymes. And no one told me. No one. I’m so tired of being blamed for my own biology. This post? It felt like someone finally saw me.
Also, I started taking St. John’s wort because I thought it was 'natural' and safe. I didn’t know it would make my birth control useless. I got pregnant. I had a miscarriage. I didn’t tell anyone. I still don’t. But I wish I had. I wish someone had told me. Please-tell your friends. Tell your mom. Tell your sister. Supplements aren’t harmless. They’re silent killers.
Ian Long
I get the gene stuff, but let’s not pretend this is new. My grandpa was on warfarin in the 70s. They adjusted his dose by watching his gums bleed. We didn’t need DNA to know he was sensitive-we just paid attention. The real problem isn’t genetics. It’s that doctors don’t listen anymore. They click boxes. They trust algorithms. They don’t sit with patients and ask, 'What happened after you took this?'
My aunt got hospitalized for a lithium overdose because her doctor didn’t check her kidney function. She was 72. They gave her the same dose as a 30-year-old. She didn’t have a gene variant. She had a lazy doctor. Fix that first.
Elisha Muwanga
Another woke medical article. Next you’ll tell me my skin color affects my pain tolerance. Genetic testing? In a country where people can’t get basic care? This isn’t science-it’s virtue signaling with a lab coat. Let’s fix access to penicillin before we hand out $250 gene reports.
Jerian Lewis
I’ve had 3 different antidepressants fail. None worked. I didn’t know why. Now I know-I’m a CYP2D6 ultra-rapid metabolizer. I used to think I was broken. Turns out I’m just genetically optimized for ignoring pills.
Phil Kemling
If we’re talking about individual variation, then why do we still treat the body like a machine with fixed inputs? The real question isn’t ‘why do drugs affect people differently?’ It’s ‘why do we keep trying to force biology into a one-size-fits-all model?’ Maybe the problem isn’t the drug. Maybe it’s the assumption that all humans are the same.
Drew Pearlman
Hey, I just want to say-you’re not alone. I’ve been where you are. I took metoprolol for hypertension and felt like I was drowning in slow motion. My doctor said 'give it time.' I almost quit. But then I got tested. Turns out, I’m a CYP2D6 poor metabolizer. They switched me to a different beta-blocker and I felt like a new person. I’m alive because I asked. Please, if you’ve ever felt weird on a med-ask. Don’t suffer in silence. Your body isn’t broken. It’s just speaking a different language. And now we’re learning how to listen.
Patty Walters
just got my results back-CYP2C19 poor metabolizer. i’ve been on clopidogrel for 2 years. no idea it wasn’t working. my cardiologist never mentioned it. now i’m on prasugrel. life changed. also, stop taking turmeric with blood thinners. i learned that the hard way. ps: pharmacist saved me. talk to yours.